Calm your bladder.
Calm your mind.
Live Without Limits
Patients with bladder dysfunction deserve a simple treatment option that doesn't involve drugs or invasive surgeries so they can return to doing what they love with confidence.
Whether it's a long car trip, a night out with friends, or simply sleeping through the night, bladder control shouldn't hold you back from living fully.
A new way forward
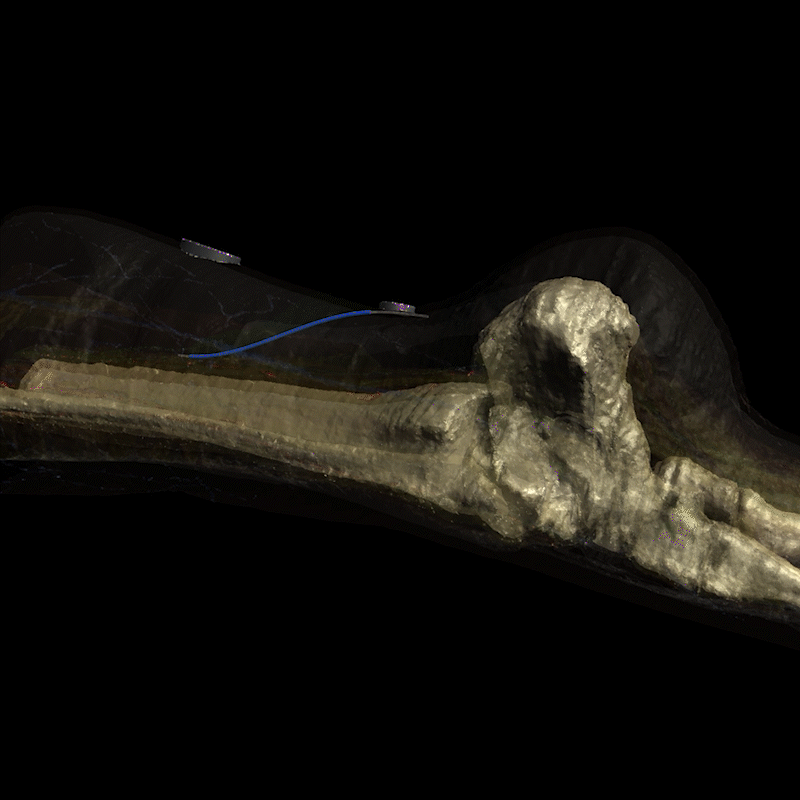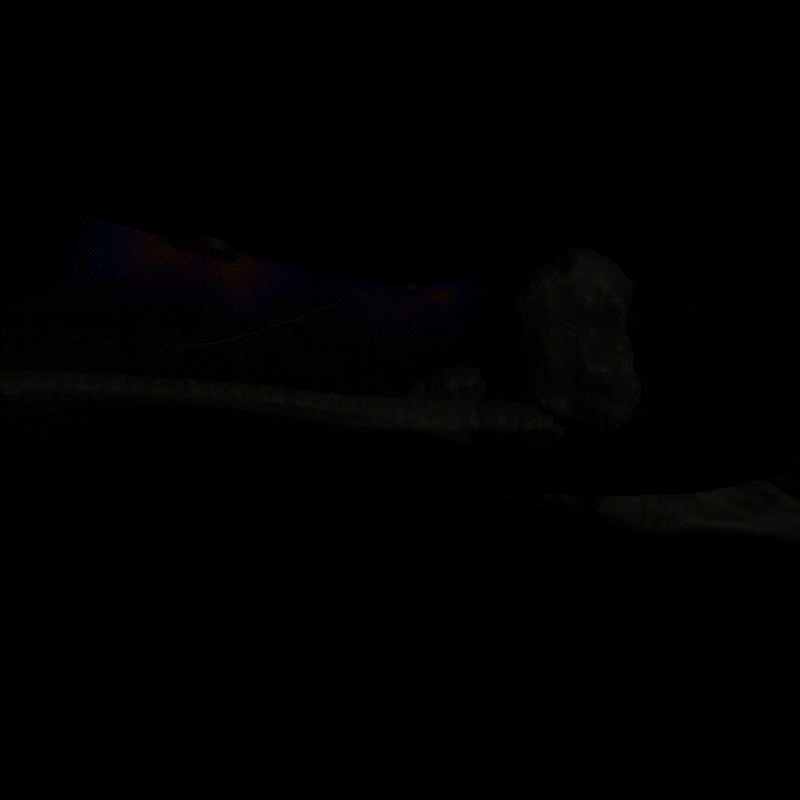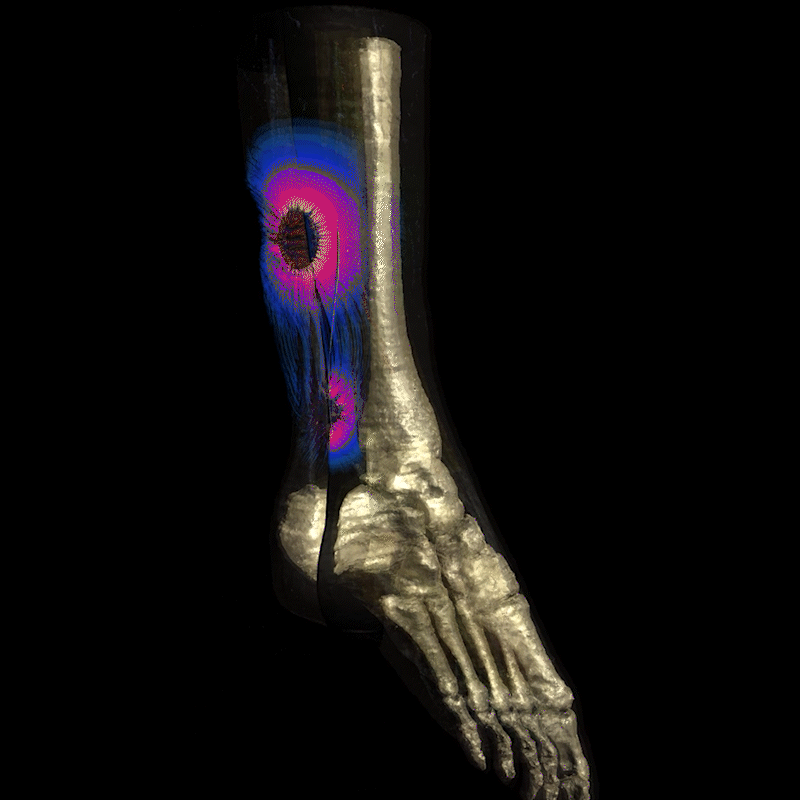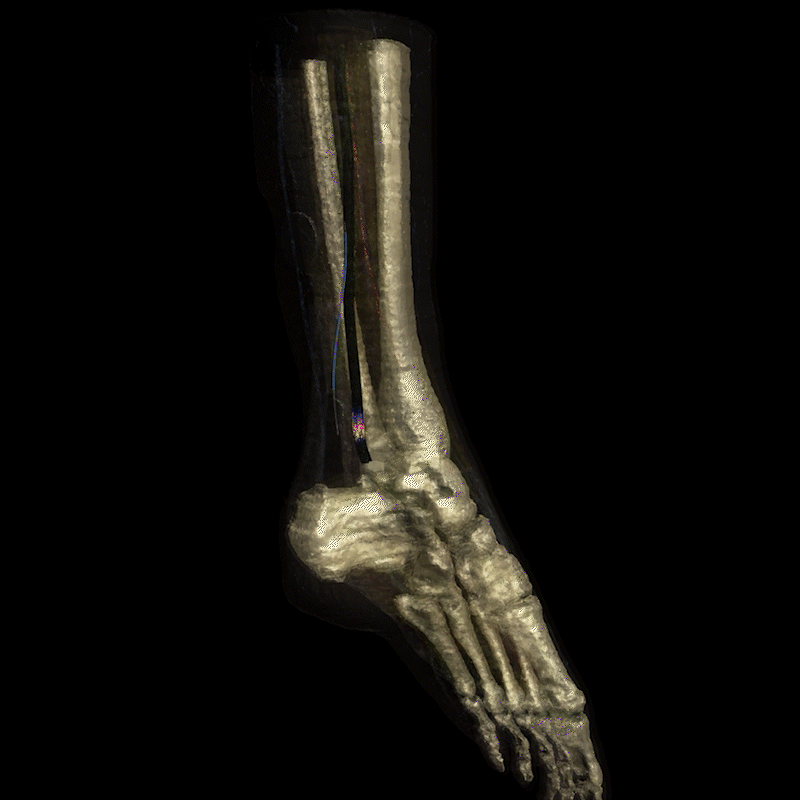
Calm Medical uses the Injectrode® platform to deliver precise tibial and sacral nerve stimulation with the vision of treating neurogenic bladder and overactive bladder conditions through an elegant needle-based procedure.
Current options force patients to choose between medications with side effects or complex surgical procedures with limited minimally invasive alternatives.
Our injectable neuromodulation system delivers customizable electrical therapy without surgery - making effective treatment accessible to more patients as an early option, not a last resort.
Why Injectrode?
Precision
Targeting
The Injectrode creates a conductive path for the electrical current to travel to the nerve target, delivering precise stimulation exactly where it's needed rather than a dispersed field.
Edema
Challenges
Patient edema increases the distance from skin to nerve, making surface-based TENS stimulation ineffective. Our approach places the electrode directly at the nerve target, bypassing the variable tissue thickness entirely.
Consistent Nerve Activation
Unlike TENS, which can vary dramatically based on skin conditions and placement, the Injectrode provides reliable, consistent nerve activation with predictable therapeutic outcomes.
Our mission is to lower the barriers of neuromodulation treatments for patients, physicians, providers, and payers by transforming an invasive surgery into an elegant needle-based intervention — making it an early choice, not a last resort.
As a wholly owned subsidiary of Neuronoff Inc., Calm Medical leverages years of neuromodulation research to address the unmet needs for bladder dysfunction treatment.
Regulatory Pathway: Our Injectrode platform enables a 510(k) and 510(k) de novo submission pathway as a Class II device, with pre-submission planned for 2026 and FDA submission targeted for 2027.
Pioneering Accessible Neuromodulation
Image: Realistic simulation of electrical current field propagation through the leg via Injectrode, and action potential initiation on the tibial nerve is provided by Sim4Life®. Sim4Life is a FDA-recognized Medical Device Development Tool (MDDT) for use in developing and testing medical devices, specifically for evaluating the safety of active implantable medical devices (AIMDs) during MRI scans and to simulate neural activation probabilities. More information can be found on the ZMT website (https://zmt.swiss/ and https://sim4life.swiss/.)
How does neurostimulation treat bladder dysfunction?
Tibial nerve stimulation works by modulating the neural pathways that control bladder function. The tibial nerve shares nerve roots with the nerves that control bladder and pelvic floor function. When stimulated, it sends signals through the spinal cord to the brain, helping to:
Normalize bladder contractions in overactive bladder
Reduce urgency and frequency symptoms
Enhance bladder capacity and control
This neuromodulation approach addresses the root neurological dysfunction rather than just masking symptoms, offering patients a pathway to restored bladder control.
Upcoming Neurogenic Bladder Study
Neuronoff (Calm Medical) recently secured significant federal support for advancing bladder neuromodulation. The Department of Defense awarded $1.73 million for a comprehensive three-year clinical trial evaluating our system for neurogenic bladder management in spinal cord injury patients.
This groundbreaking study, conducted in partnership with the University of Texas Health Science Center at San Antonio, represents the first investigation of our minimally invasive approach for neurogenic bladder treatment—a condition affecting over 80% of spinal cord injury patients.